Which Metals Form Cations With Varying Positive Charges
Ca 2 ChromiumII Cr 2 Chromous. 34 protons 45 neutrons.
Solved Question 1 Which Metals Form Cations With Varying Chegg Com
In the chemistry of the transition elements the 4s orbital behaves as the outermost highest energy orbital.

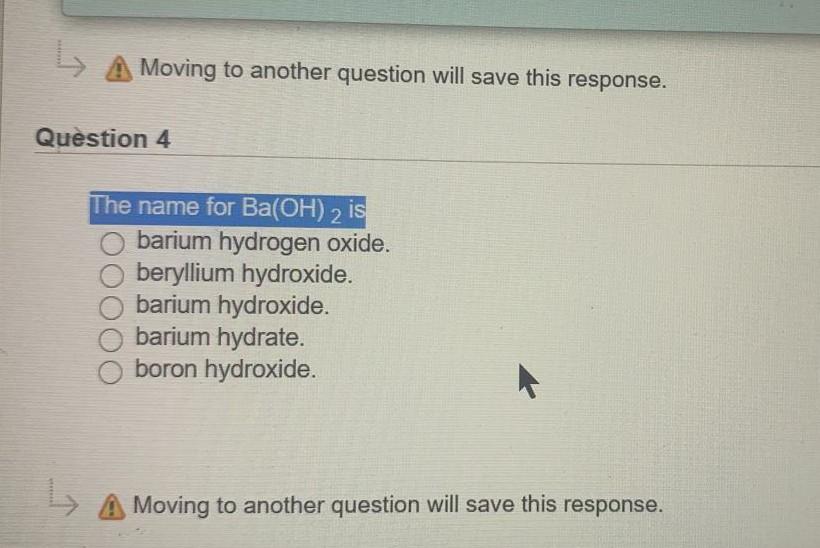
. The correct name for N3- is. Up to 24 cash back Most transition metals 3-12 and Group 4A 14 metalsform 2 or more positive ions except Zn2Ag and Cd2 which form only one ion. Which metals form cations with varying positive.
The positive ion the cation is the atom of a metallic element that loses one electron or more during the chemical reactions The positive ion carries positive charges equal. Which of the following is incorrect. The correct name for LiCl is.
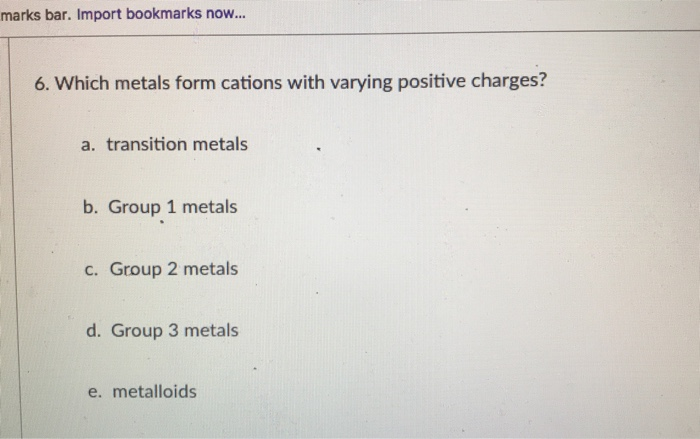
5 rows See the answer See the answer done loading. Question 1 Which metals form cations with varying positive charges. Group 3 metals C.
Which metals form cations with varying positive charges. Which metals form cations with varying positive charges. Chapter 02 - Atoms Molecules and Ions.
Transition Metal Ions. NH 4 Barium. They are formed when a metal loses its electrons.
34 protons 79 neutrons 2 electrons. When these metals form ions the 4s electrons are always lost first. A Ions are formed by adding electrons to a neutral atom.
As a result the magnesium ion and the two ions. Group 1 metals c. Virtually all of the transition metals form dipositive 2cations along with one.
Most transition metals form multiple cations that is they have more than one possible amount of positive charge. A species with 12 protons and. Based on the following observations for 3.
B Ions are formed by changing the number of protons in an atoms nucleus. However the outermost s electrons are always the first to be removed in the process. C Oxidization State of Hydrogen generally is 1 in compounds.
Most transition metals differ from the metals of Groups 1 2 and 13 in that they are capable of forming more than one cation with different ionic charges. The alkaline earth metals IIA elements lose two electrons to form a 2 cation. A Moving to another question will save this response.
Cations are positively charged ions. The two atoms of the other element each form a 1 charge. Group 3 metals e.
They lose one or more than one electron and do not lose any protons. Group 1 metals E transition metals Moving to another question will save this response. ChromiumIII Cr 3 Chromic.
The alkali metals the IA elements lose a single electron to form a cation with a 1 charge. A transition metals B Group 1 metals C Group 2 metals D Group 3 metals E metalloids. Electron Transfer Essay.
C Ions are formed by removing electrons from a. The main group metals tend to form salts such as NaCl Mg 3 N 2 and CaS in which there are just enough negative ions to balance the charge on the positive ions. Therefore they possess a net.
D Elements in Group 15 have an oxidation number of 3 in binary metal compounds with metals. Group 2 metals B. AP Chemistry Review - Atoms Molecules and Ions.
CaO is calcium oxide cNH4NO3 is ammonium nitrate dK3PO4 is potassium phosphate eMgSO3 is magnesium sulfite. According to the Aufbau process the electrons fill the 4 s sublevel before beginning to fill the 3 d sublevel. Group 2 metals d.
Which of these is the correct number of particles in the nuclide above.

Solved When The Following Equation Is Balanced The Chegg Com
Solved Marks Bar Import Bookmarks Now 6 Which Metals Chegg Com

Solved Question 1 Which Metals Form Cations With Varying Chegg Com

Solved Question 1 Which Metals Form Cations With Varying Chegg Com
No comments for "Which Metals Form Cations With Varying Positive Charges"
Post a Comment